In recent years, the domain of medicine has witnessed some groundbreaking advances through the emergence of Cell and Gene Therapies (CGT). These innovative treatments hold the potential to revolutionise healthcare by harnessing the power of living cells and genetic engineering to combat diseases at their source. The promise they bring with them is immense, offering new hope to patients with conditions that were once deemed incurable.
However, while CGTs present a beacon of optimism, their end-to-end operation is riddled with multifaceted challenges impacting supply chain, manufacturing and quality. In this first series of articles, we embark on a journey through the complexities of the CGT supply chain, exploring the hurdles that pharmaceutical companies face and the ingenious solutions that are reshaping the landscape of modern medicine. The next series will address the manufacturing challenges and the unique nature of the CMC burden.
A unique aspect of CGTs is their patient-specific nature and personalised therapeutic approach
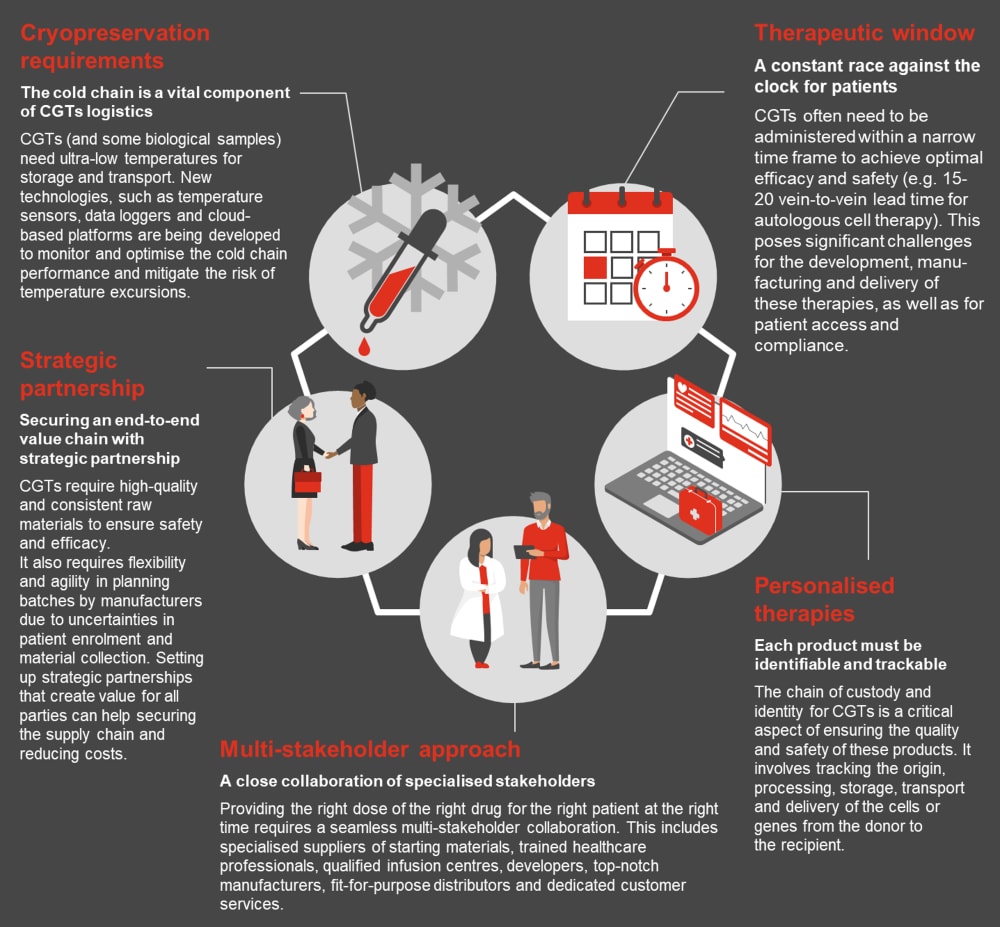
Patient populations are limited, and treatment is often one-time only within a short therapeutic window of opportunity. For example, autologous cell therapies are meticulously tailored to individual patients. While this personalised approach enhances therapeutic effectiveness, it introduces complexities. These therapies often have vein-to-vein lead times of 15 to 20 days, significantly affecting patient eligibility. The potential risk of batch termination looms large, which not only adds costs but also causes emotional distress for patients and their families.
Maintaining the delicate balance between personalisation and timely treatment delivery is a complex challenge with profound implications for both patients, manufacturers and distributors. Strategies such as predictive modelling and streamlined logistics can help mitigate patient-specific challenges, ensuring that therapies reach patients when needed while minimising the costs associated with batch failures. Real-time tracking of the manufacturing process until the supply to the healthcare facility via a digital portal will maximise communication across all parties, including the patient at the centre of the journey.
A patient-centric supply chain approach is key for CGT’s operation
We see many challenges and complexities to overcome when setting up a reliable and flexible supply chain to deliver advanced therapies to patients. In contrast to the conventional linear pharmaceutical supply chains, advanced personalised therapies necessitate supply chains centred around the patient. This entails including caregivers and clinical operations in the end-to-end production process, starting with the initial collection of materials to the administration to the patient. Maintaining a strict chain of custody and preserving the chain of identity ensure the safe and prompt delivery of treatments to the right patients. The adoption of new tracking technologies guarantee that the products are closely monitored at each stage of the manufacturing process.
One key success factor is to know your product, start preparing early and be sure to think ahead. Ensure that the supply chain is tailored to the product and based on good risk/benefit management. This requires collaboration with development departments early on to understand the requirements and limitations of the product across the value chain.
Additionally, the process of scheduling manufacturing slots at Contract Development and Manufacturing Organisations (CDMOs) necessitates an additional level of coordination, encompassing healthcare professionals and the admission of patients to treatment centres. Given the often limited shelf life of the products, it is imperative to closely oversee the production, shipping, patient scheduling and treatment administration in order to guarantee interconnections across the end-to-end patient journey.
An optimised supply chain is crucial for the timely and cost-effective production of CGTs. Delays or disruptions can have dire consequences for both patients and manufacturers. Digitalisation and real-time tracking technologies can minimise supply chain risks by enhancing product visibility across the value chain. Furthermore, strategic partnerships with suppliers can ensure a reliable supply of critical materials, reducing the risk of shortages and associated costs.
An integrated, seamless and safe supply chain, including high-quality cryopreservation technologies, can safeguard access to patients
The delicate nature of living cells requires 1) adapted cryopreservation technologies and media, and 2) precise temperature control during storage and transportation (i.e. liquid nitrogen). The cold chain minimises the risk of degradation, contamination or loss of potency of these therapies, which can affect their efficacy and safety for patients. By investing in temperature-controlled packaging and monitoring, pharmaceutical companies can ensure that these therapies reach patients in optimal condition. This not only safeguards the effectiveness of the treatment but also reduces the risk of costly batch failures due to compromised product integrity.
Advanced temperature monitoring technologies, such as IoT-based sensors, are increasingly being employed to ensure the integrity of the cold chain, reducing the risk of costly losses. Collaborative efforts between pharmaceutical companies and logistics providers are essential to ensure the seamless operation of the cold chain. Additionally, the use of data analytics can predict potential cold chain disruptions, allowing for proactive interventions and cost savings. Manufacturing at the point-of-care can also help to mitigate the risks of long-distance transport between delocalised facilities and qualified treatment centres, reducing complexities in the supply chain.
CGTs require the rigid and linear supply chain to be pivoted towards a flexible and responsive patient-centric model to maximise the access and safety of the therapy
Looking ahead
In conclusion, the supply chain for cell and gene therapies is a complex and dynamic system that involves multiple stakeholders, processes and technologies. By embracing a patient-centric mindset and adopting novel technologies and platforms, such as automation, digitalisation, analytics, artificial intelligence and blockchain, pharmaCos will enhance the efficiency, quality, traceability and security of the supply chain. Looking ahead, the future of CGTs’ supply chain will require a new operating model and the diversification of business models and strategies, such as outsourcing, contracting, licensing, partnering and co-development to share risks and costs, leverage capabilities and resources and create value for all parties involved in the supply chain.






